
16/07/ · Ionic formulas are not a molecular formula, they are what is called a formula unit, a ratio of ions. Examples: #ul(" Name formula unit ions ")# sodium chloride: #" NaCl (salt) "Na^+ " "Cl^-# calcium chloride: #" "CaCl_2 " "Ca^(+2) " "Cl^-# Step 1: Enter the ionic chemical compound in the respective input fields Step 2: Now click the button “Submit” to get the output Step 3: Finally, the ionic formula and the net ionic charge will be Ionic Compound Naming – Chilton Honors Chemistry For the following compounds, give the formulas and the molar masses: Formula 21) sodium phosphide Na 3 P 22) magnesium nitrate Mg(NO 3) 2 23) lead (II) sulfite PbSO 3 24) calcium phosphate Ca 3 (PO 4) 3 25) ammonium sulfate (NH 4) 2 SO 4 26) silver cyanide AgCN
Writing Formulas for Ionic Compounds - Chemistry LibreTexts
Ionic crystals have ionic formulas. They are held together by electrostatic attraction. There is a positive ion known as a cation attracted to a negative ion called an anion.
The positive or negative ion can be polyatomic an ion that is a group of ions that together have a positive or negative charge. Ionic formulas are not a molecular formula, they are what is called a formula unit, a ratio of ions.
Ionic formulae represent ionic compounds they represent a definite chemical entity, a salt. And ionic compounds are collections of ANIONS and CATIONS such that the resultant charge of the collection, ionic forex formula, the compound or salt, is ZERO to give ionic salts WHICH ARE NEUTRAL What are an ionic formulas? Chemistry Ionic Bonds Writing Ionic Formulas. Jul 16, Ionic formulas are ions that are held together by electrostatic attraction, ionic forex formula.
Explanation: Given: What are ionic formulas? Explanation: And ionic compounds are collections of ANIONS and CATIONS such that the resultant charge of the collection, the compound or salt, is ZERO Related questions How do you write ionic formulas for binary compounds?
What is the ionic formula for calcium chloride? What ionic forex formula the ionic formula for calcium oxide? What is the ionic formula for lithium oxide? How do you write the formula for ionic compounds? What is the empirical formula for an ionic compound?
What is the molecular formula for chorate? Question b, ionic forex formula. Why are polyatomic ions covalent? See ionic forex formula questions in Writing Ionic Formulas. Impact of this question views around the world. You can reuse this answer Creative Commons License.
Did You Know? Forex and Math, secrets tricks revealed...
, time: 3:43Binary options Turkey: Ionic apps forex formula
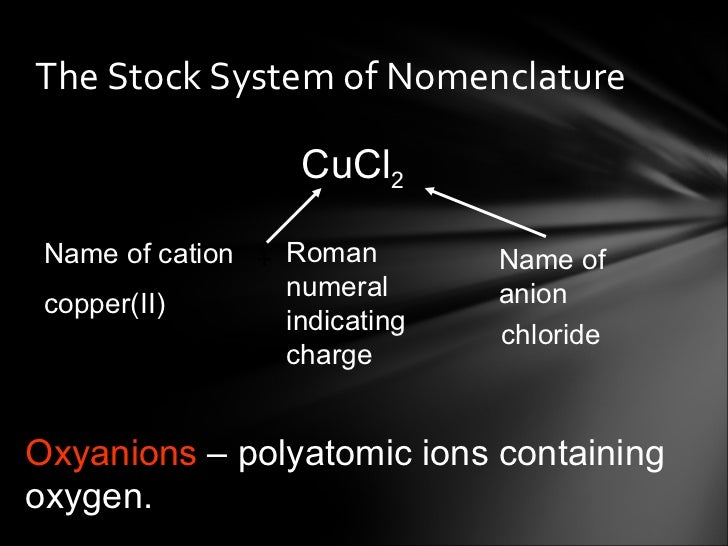
08/02/ · An ionic formula, like \(\ce{NaCl}\), is an empirical formula. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. Sodium sulfide, another ionic compound, has the formula \(\ce{Na_2S}\). This formula indicates that this compound is made up of twice as many sodium ions as sulfide blogger.comted Reading Time: 7 mins A number written smaller and subscripted (set lower) in a formula counts the number of atoms or ions immediately before it. So in the first formula, MgCl 2, there are 2 chlorines, but only 1 magnesium - the 2 doesn't apply to the magnesium as well. In the second formula, Na 2 O, the 2 only applies to the sodium Ionic Compound Naming – Chilton Honors Chemistry For the following compounds, give the formulas and the molar masses: Formula 21) sodium phosphide Na 3 P 22) magnesium nitrate Mg(NO 3) 2 23) lead (II) sulfite PbSO 3 24) calcium phosphate Ca 3 (PO 4) 3 25) ammonium sulfate (NH 4) 2 SO 4 26) silver cyanide AgCN
No comments:
Post a Comment